Recommended Global Medical Webinars & Conferences
Europe & UK
Asia Pacific & Middle East
Canada
Medical Toxicology Pharma 2020
About Conference
World Congress on Medical Toxicology is scheduled on December 01-02, 2020 Kyoto, Japan. It offers a unique platform to present and know the latest updates with a complete approach to diverse areas of interest. As said by Paracelsus (1493-1541) father of Toxicology’ goes “All things are with poison and nothing is without poison” only the dose can make a thing not poisonous”. Toxicology deals with the study of the correlation between the dose and its subsequent therapeutic effect. The therapeutic effect can convert to toxic effect with the increase in the level of dose. Toxicological studies starts right from the preclinical drug development. These studies are supported by advanced techniques such as In vivo imaging.
Conference Series llc LTD organizes a conference series of 1000+ Global Events inclusive of 300+ Conferences, 500+ Upcoming and Previous Symposiums and Workshops in USA, Europe & Asia with support from 1000 more scientific societies and publishes 700+ Open access journals which contains over 30000 eminent personalities, reputed scientists as editorial board members.
Why MEDICAL TOXICOLOGY PHARMA 2020??
Medical Toxicology Pharma 2020 provides a global platform to meet and develop interpersonal relationship with the world’s leading toxicologists, pharmacologists, clinical research professionals, biochemists and also the industrialists who will provide you with the latest innovations and scientific approaches thus help you in widening your scientific knowledge.
The topics of the Congress will reflect the recent advances, current trends, future trends and new approaches in Toxicology and Pharmacology. The congress will cover among others: Mechanisms and modes of action of various toxins, Clinical and Forensic toxicology, Emerging in vitro models, Medicine Development and Safety Testing, Regulatory toxicology and last but not least, a broad scope of pharmacology and toxicology in different fields. Thus, our objective is, to create platforms which will gather eminent scientists who will undoubtedly enrich our congress during the Q&A sections.
Target Audience:
- Academicians including Professors
- PhD Scholars
- Students carrying out laboratory and field studies
- Pharmaceutical Industrial Giants
- Toxicology Societies and the people Associated
- Nobel laureates in Health Care and Medicine
- Pharmacists
- Pharmacologists
- Toxicology Professionals
- Genetic Professionals
- Pathology Professionals
- Forensic Professionals
- Pharmaceutical companies
- Clinical Laboratories and Technicians
- Bio-informatics Professionals
- Research Institutes and members
- Supply Chain companies
- Manufacturing Companies
- Training Institutes
- Business Entrepreneurs
Sessions/Tracks
Track 1: Toxicology
Toxicology is the scientific study of harmful effects of chemical, biological and physical agents in biological systems that establish the extent of damage in living organisms. It includes observing symptoms, mechanisms, detection and treatments of toxic substances, in certain relation to the poisoning of humans. It consists of environmental agents and chemical compounds, as well as Pharmaceutical Compounds that are manufactured for medical use by humans. These substances may produce toxic effects in living organisms including disturbance in growth patterns, discomfort, disease and death. Toxicology is the qualitative and quantitative study of the adverse effects of chemicals and other materials on living organisms. The dose of the substance is an important factor in toxicology, as it has a relationship with the effects on the individual. Factors that influence toxicity include the dose, the route of exposure, shape and structure of the chemical, the species, individual human factors and environment. Toxicology and Pharmacology are both studies that involve in assessing the properties of chemicals and their actions on the body, but differ significantly in other areas. Pharmacology focuses on the therapeutic effects of pharmaceutical substances and how they can be used most effectively for medical purpose, whereas, toxicology is closely related to the adverse effects that can occur in living organisms that come into contact with chemical compounds. Toxicologists are also concerned with determining the risk of certain substances with risk assessment tools.
Related Conferences: Toxicology Conferences | Pharmacology Conferences | Pharmaceutical Conferences
20th World Congress on Toxicology and Pharmacology, May 06-07, 2019 Tokyo, Japan; 16th Global Summit on Toxicology and Applied Pharmacology, October 15-16, 2018 Las Vegas, Nevada, USA; 15th Euro-Global Summit on Applied Pharmacology and Toxicology, July 02-04, 2018 Berlin, Germany; 17th Global Toxicology and Risk Assessment Conference, October 22-24, 2018 Budapest, Hungary; 14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, Sweden; International Conference on Toxicology and Risk Assessment, March 20-22, 2019 Frankfurt, Germany; Euro Summit on Toxicology and Pharmacology, November 19-21, 2018 Rome, Italy; 54th Congress of the European Societies of Toxicology 2018, September 02-05, 2018 Brussels, Belgium; Society of Environmental Toxicology and Chemistry (SETAC); IUTOX: International Union of Toxicology; EUROTOX; ABD: American Board of Toxicology
Related Societies
Europe: Society of Environmental Toxicology and Chemistry, Federation of European Toxicologists & European Societies of Toxicology (EUROTOX), British Toxicology Society (BTS), European Society of Toxicology In-Vitro.
USA : Society of Toxicology, International Association of Therapeutic Drug Monitoring and Clinical Toxicology, Fellow of the American Association for the Advancement of Science, Society of toxicologic pathology.
Asia pacific and Middle East: The Asian Society of Toxicology (ASIATOX), Asia Pacific Association of Medical Toxicology (APAMT), Chinese Society of Toxicology, Society of Toxicology India (STOX).
Track 2: Analytical Toxicology
Analytical toxicology is the detection, identification, and measurement of foreign compounds in biological and other specimens. Analytical methods are available for a wide range of compounds like chemicals, pesticides, drugs and natural toxins. Analytical toxicology is used in the diagnosis, management, prognosis, and prevention of poisoning. Analytical toxicology laboratories may be involved in activities such as the assessment of exposure following chemical incidents, therapeutic drug monitoring, monitoring the drugs of abuse and forensic analyses. They may also be involved in research like determining the pharmacokinetic and toxicokinetics property. Liquid chromatography-mass spectrometry and Gas chromatography-mass spectrometry are generally used today in analytical toxicology. While planning the development of an analytical toxicology service there is a number of considerations. These comprise the pattern of poisoning and, therefore, the specific substances for which analyses will be required, the existing infrastructure, the availability of ongoing technical support, spare parts and reagents from suppliers, the availability of a team of trained staff and the capacity to train new staff and provide continuing professional development.
Related Conferences: Toxicology Conferences | Pharmacology Conferences | Pharmaceutical Conferences
20th World Congress on Toxicology and Pharmacology, May 06-07, 2019 Tokyo, Japan; 16th Global Summit on Toxicology and Applied Pharmacology, October 15-16, 2018 Las Vegas, Nevada, USA; 15th Euro-Global Summit on Applied Pharmacology and Toxicology, July 02-04, 2018 Berlin, Germany; 17th Global Toxicology and Risk Assessment Conference, October 22-24, 2018 Budapest, Hungary; 14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, Sweden; International Conference on Toxicology and Risk Assessment, March 20-22, 2019 Frankfurt, Germany; Euro Summit on Toxicology and Pharmacology, November 19-21, 2018 Rome, Italy; 54th Congress of the European Societies of Toxicology 2018, September 02-05, 2018 Brussels, Belgium; Society of Environmental Toxicology and Chemistry (SETAC); IUTOX: International Union of Toxicology; EUROTOX; ABD: American Board of Toxicology
Related Societies
Europe: Society of Environmental Toxicology and Chemistry, Federation of European Toxicologists & European Societies of Toxicology (EUROTOX), British Toxicology Society (BTS), European Society of Toxicology In-Vitro.
USA : Society of Toxicology, International Association of Therapeutic Drug Monitoring and Clinical Toxicology, Fellow of the American Association for the Advancement of Science, Society of toxicologic pathology.
Asia pacific and Middle East: The Asian Society of Toxicology (ASIATOX), Asia Pacific Association of Medical Toxicology (APAMT), Chinese Society of Toxicology, Society of Toxicology India (STOX).
Track 3: Genotoxicity
Genotoxicity describes the property of chemical compounds that damages the genetic information within a cell causing mutations, which may lead to cancer. Genotoxic chemicals exert their adverse effect through interaction with genetic material (DNA) of cells. Genotoxicity testing of new chemical entities is an integral part of the drug development process and is a regulatory necessity prior to the approval of new drugs. To assay for Genotoxic molecules, researchers assay for DNA damage in cells exposed to the toxic substrates. Techniques like in vitro and in vivo Toxicology Tests, Ames Assay and Comet Assay have been developed to determine the chemicals' potential to cause DNA damage that may lead to cancer. Genotoxicity tests are designed to detect drugs which can cause genetic damage directly or indirectly by various mechanisms of action. Compounds which are identified as Genotoxic in these tests have the ability to be human carcinogens and ultimately may induce cancer and heritable defects. Genotoxic testing of new chemical entities is an integral part and is a regulatory requirement prior to the approval of new drugs, in Drug Development process. Late stage failures can reduced by identifying genotoxicity at an early stage in Drug Discovery rather than during regulatory assessment.
Related Conferences: Toxicology Conferences | Pharmacology Conferences | Pharmaceutical Conferences
20th World Congress on Toxicology and Pharmacology, May 06-07, 2019 Tokyo, Japan; 16th Global Summit on Toxicology and Applied Pharmacology, October 15-16, 2018 Las Vegas, Nevada, USA; 15th Euro-Global Summit on Applied Pharmacology and Toxicology, July 02-04, 2018 Berlin, Germany; 17th Global Toxicology and Risk Assessment Conference, October 22-24, 2018 Budapest, Hungary; 14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, Sweden; International Conference on Toxicology and Risk Assessment, March 20-22, 2019 Frankfurt, Germany; Euro Summit on Toxicology and Pharmacology, November 19-21, 2018 Rome, Italy; 54th Congress of the European Societies of Toxicology 2018, September 02-05, 2018 Brussels, Belgium; Society of Environmental Toxicology and Chemistry (SETAC); IUTOX: International Union of Toxicology; EUROTOX; ABD: American Board of Toxicology
Related Societies
Europe: Society of Environmental Toxicology and Chemistry, Federation of European Toxicologists & European Societies of Toxicology (EUROTOX), British Toxicology Society (BTS), European Society of Toxicology In-Vitro.
USA : Society of Toxicology, International Association of Therapeutic Drug Monitoring and Clinical Toxicology, Fellow of the American Association for the Advancement of Science, Society of toxicologic pathology.
Asia pacific and Middle East: The Asian Society of Toxicology (ASIATOX), Asia Pacific Association of Medical Toxicology (APAMT), Chinese Society of Toxicology, Society of Toxicology India (STOX).
Track 4: Forensic Toxicology
Forensic toxicology uses toxicology and other disciplines such as analytical chemistry, clinical chemistry and pharmacology to aid medical or legal investigation of drug use, poisoning and death. It deals with the investigation of toxic substances, poisonous products and environmental chemicals. The primary concern of Forensic Toxicology is to obtain and interpret the results. Toxicological analysis can be done to various kinds of samples. Forensic toxicology involves not only determining the presence of Toxic substance in the post-mortem body, but how the body’s natural processes affect the substance, including chemical change and dilution. Forensic toxicologists perform scientific tests on tissue samples and bodily fluids to identify any chemicals or drugs present in the body. The choice of method for testing is highly depends on the material on which the testing is performed and what kind of substance one expects to find. Analytical methods used in forensic toxicology should be carefully tested by performing a validation of the method to ensure definite results at all times. Forensic toxicologists also work on cases involving environmental contamination, to determine the impact of chemical spills on living organisms. Investigators rely on the forensic toxicologist to make reliable conclusions about the impact a specific amount of a specific substance would have on a specific individual. Currently, forensic toxicology is the study of drugs, alcohol and poisons, including their chemical composition, identification and preparations. It comprises knowledge about the absorption, distribution and elimination process of such substances in the body, as well as the manner in which the body responds to their presence and the factors which define Drug Safety and efficacy.
Related Conferences: Toxicology Conferences | Pharmacology Conferences | Pharmaceutical Conferences
20th World Congress on Toxicology and Pharmacology, May 06-07, 2019 Tokyo, Japan; 16th Global Summit on Toxicology and Applied Pharmacology, October 15-16, 2018 Las Vegas, Nevada, USA; 15th Euro-Global Summit on Applied Pharmacology and Toxicology, July 02-04, 2018 Berlin, Germany; 17th Global Toxicology and Risk Assessment Conference, October 22-24, 2018 Budapest, Hungary; 14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, Sweden; International Conference on Toxicology and Risk Assessment, March 20-22, 2019 Frankfurt, Germany; Euro Summit on Toxicology and Pharmacology, November 19-21, 2018 Rome, Italy; 54th Congress of the European Societies of Toxicology 2018, September 02-05, 2018 Brussels, Belgium; Society of Environmental Toxicology and Chemistry (SETAC); IUTOX: International Union of Toxicology; EUROTOX; ABD: American Board of Toxicology
Related Societies
Europe: Society of Environmental Toxicology and Chemistry, Federation of European Toxicologists & European Societies of Toxicology (EUROTOX), British Toxicology Society (BTS), European Society of Toxicology In-Vitro.
USA : Society of Toxicology, International Association of Therapeutic Drug Monitoring and Clinical Toxicology, Fellow of the American Association for the Advancement of Science, Society of toxicologic pathology.
Asia pacific and Middle East: The Asian Society of Toxicology (ASIATOX), Asia Pacific Association of Medical Toxicology (APAMT), Chinese Society of Toxicology, Society of Toxicology India (STOX).
Track 5: Food Safety and Environmental Toxicology
Food safety is a discipline which describes preparation, handling and storage of food in ways that avoid foodborne illness. This involves a number of routines that should be followed to avoid potentially severe health hazards. Food safety includes the origins of food including the practices relating to food labelling, food additives, pesticide residues and food hygiene as well as policies on Biotechnology and food. Food can transmit disease from person to person as well as help as a growth medium for bacteria that can cause food poisoning. In developed countries there are sophisticated standards for food preparation, whereas in lesser developed countries the main issue is simply the availability of sufficient safe water, which is usually a critical item. Environmental toxicology is a field of science concerned with the study of the harmful effects of various physical, chemical and biological agents on living organisms. Harmful effects of biological and chemical agents can include toxicants from pesticides, pollutants, fertilizers and insecticides all of which can impact an organism and its population through shifts in species diversity and abundance. Living organisms can be exposed to Toxicants at various stages of their life cycle. The amount of toxicity can vary depending on where the organism is found within its food web.
Related Conferences: Toxicology Conferences | Pharmacology Conferences | Pharmaceutical Conferences
20th World Congress on Toxicology and Pharmacology, May 06-07, 2019 Tokyo, Japan; 16th Global Summit on Toxicology and Applied Pharmacology, October 15-16, 2018 Las Vegas, Nevada, USA; 15th Euro-Global Summit on Applied Pharmacology and Toxicology, July 02-04, 2018 Berlin, Germany; 17th Global Toxicology and Risk Assessment Conference, October 22-24, 2018 Budapest, Hungary; 14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, Sweden; International Conference on Toxicology and Risk Assessment, March 20-22, 2019 Frankfurt, Germany; Euro Summit on Toxicology and Pharmacology, November 19-21, 2018 Rome, Italy; 54th Congress of the European Societies of Toxicology 2018, September 02-05, 2018 Brussels, Belgium; Society of Environmental Toxicology and Chemistry (SETAC); IUTOX: International Union of Toxicology; EUROTOX; ABD: American Board of Toxicology
Related Societies
Europe: Society of Environmental Toxicology and Chemistry, Federation of European Toxicologists & European Societies of Toxicology (EUROTOX), British Toxicology Society (BTS), European Society of Toxicology In-Vitro.
USA : Society of Toxicology, International Association of Therapeutic Drug Monitoring and Clinical Toxicology, Fellow of the American Association for the Advancement of Science, Society of toxicologic pathology.
Asia pacific and Middle East: The Asian Society of Toxicology (ASIATOX), Asia Pacific Association of Medical Toxicology (APAMT), Chinese Society of Toxicology, Society of Toxicology India (STOX).
Track 6: Toxicology Applications
Application of procedures and principles of toxicology to prevent adverse health effects from drug candidates. To estimate the safety of potential drug candidates in the drug development process is the primary objective of toxicology studies. This can be done by using relevant animal models and validated procedures. The ultimate goal is to translate the animal model responses into an understanding of the risk for human subjects. To this end, the toxicologist must be conscious of the international guidelines for safety evaluation, as well as traditional and non-traditional toxicology models. Toxicology profile consists of safety Pharmacology, acute and sub chronic toxicology, chronic toxicology, ADME studies, genetic toxicology, reproductive and developmental toxicology and an evaluation of carcinogenic potential. The development of novel drugs requires non-clinical safety studies to be performed on candidate drug compounds. Such studies typically assess general toxicology safety pharmacology and Genetic Toxicity test batteries. These studies notify development of candidate drugs from the “discovery phase” through clinical development to regulatory submission and registration. Less importance was placed on the evaluation of safety issues for projects while still in the drug design phase. Therefore, this led to a number of major failures of candidate drugs in early development due to toxicological issues. In response to this costly attrition, many pharmaceutical companies have now dedicated in “Discovery-phase Toxicology” or “Discovery Safety” to detect likely hazards and to take steps to design out or significantly reduce unwanted properties at an earlier stage, with the ultimate aim of improving the probability of success in non-clinical and clinical drug development.
Related Conferences: Toxicology Conferences | Pharmacology Conferences | Pharmaceutical Conferences
20th World Congress on Toxicology and Pharmacology, May 06-07, 2019 Tokyo, Japan; 16th Global Summit on Toxicology and Applied Pharmacology, October 15-16, 2018 Las Vegas, Nevada, USA; 15th Euro-Global Summit on Applied Pharmacology and Toxicology, July 02-04, 2018 Berlin, Germany; 17th Global Toxicology and Risk Assessment Conference, October 22-24, 2018 Budapest, Hungary; 14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, Sweden; International Conference on Toxicology and Risk Assessment, March 20-22, 2019 Frankfurt, Germany; Euro Summit on Toxicology and Pharmacology, November 19-21, 2018 Rome, Italy; 54th Congress of the European Societies of Toxicology 2018, September 02-05, 2018 Brussels, Belgium; Society of Environmental Toxicology and Chemistry (SETAC); IUTOX: International Union of Toxicology; EUROTOX; ABD: American Board of Toxicology
Related Societies
Europe: Society of Environmental Toxicology and Chemistry, Federation of European Toxicologists & European Societies of Toxicology (EUROTOX), British Toxicology Society (BTS), European Society of Toxicology In-Vitro.
USA : Society of Toxicology, International Association of Therapeutic Drug Monitoring and Clinical Toxicology, Fellow of the American Association for the Advancement of Science, Society of toxicologic pathology.
Asia pacific and Middle East: The Asian Society of Toxicology (ASIATOX), Asia Pacific Association of Medical Toxicology (APAMT), Chinese Society of Toxicology, Society of Toxicology India (STOX).
Track 7: Organ Systems Toxicity
The extent to which an organ system can be damaged by a toxicant is termed as Organ System Toxicity. It is dose dependent and species specific.
Certain chemicals interfere with the body’s endocrine system resulting in adverse developmental, reproductive, neurological and immune effects. Such chemicals are termed as Endocrine Disruptors. They interfere with both human and animal endocrine systems. Endocrine Disruptors are either natural or synthetic. They may also lead to carcinogenesis. They may be otherwise called as Endocrine Disrupting Chemicals or Endocrine Disrupting compounds or Hormonally Active agents.
Related Conferences: Toxicology Conferences | Pharmacology Conferences | Pharmaceutical Conferences
20th World Congress on Toxicology and Pharmacology, May 06-07, 2019 Tokyo, Japan; 16th Global Summit on Toxicology and Applied Pharmacology, October 15-16, 2018 Las Vegas, Nevada, USA; 15th Euro-Global Summit on Applied Pharmacology and Toxicology, July 02-04, 2018 Berlin, Germany; 17th Global Toxicology and Risk Assessment Conference, October 22-24, 2018 Budapest, Hungary; 14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, Sweden; International Conference on Toxicology and Risk Assessment, March 20-22, 2019 Frankfurt, Germany; Euro Summit on Toxicology and Pharmacology, November 19-21, 2018 Rome, Italy; 54th Congress of the European Societies of Toxicology 2018, September 02-05, 2018 Brussels, Belgium; Society of Environmental Toxicology and Chemistry (SETAC); IUTOX: International Union of Toxicology; EUROTOX; ABD: American Board of Toxicology
Related Societies
Europe: Society of Environmental Toxicology and Chemistry, Federation of European Toxicologists & European Societies of Toxicology (EUROTOX), British Toxicology Society (BTS), European Society of Toxicology In-Vitro.
USA : Society of Toxicology, International Association of Therapeutic Drug Monitoring and Clinical Toxicology, Fellow of the American Association for the Advancement of Science, Society of toxicologic pathology.
Asia pacific and Middle East: The Asian Society of Toxicology (ASIATOX), Asia Pacific Association of Medical Toxicology (APAMT), Chinese Society of Toxicology, Society of Toxicology India (STOX).
Track 8: Genetic Toxicology
Genetic toxicology deals with the study of effects of different physical, chemical and biological substances on genetic material. It identifies the possible reasons for occurrence of birth defects, heritable mutations and carcinogenesis.
Mutations are caused due to genetic damage. The property of the chemical agent which causes the genetic damage is studied under genotoxicity. Mutations are the main cause for carcinogenesis.
Mutagenesis occurs spontaneously in the nature which results in the change in genetic information of an organism in a stable manner. It may also occur due to mutagens.
Genotoxic chemotherapy induces DNA damage in the cancer cells thus leading to their death. It is done by using one or more Genotoxic drugs. Damage done to a cancer is passed on to descendent cancer cells as proliferation continues. Apoptosis is induced under severe conditions.
Related Conferences: Toxicology Conferences | Pharmacology Conferences | Pharmaceutical Conferences
20th World Congress on Toxicology and Pharmacology, May 06-07, 2019 Tokyo, Japan; 16th Global Summit on Toxicology and Applied Pharmacology, October 15-16, 2018 Las Vegas, Nevada, USA; 15th Euro-Global Summit on Applied Pharmacology and Toxicology, July 02-04, 2018 Berlin, Germany; 17th Global Toxicology and Risk Assessment Conference, October 22-24, 2018 Budapest, Hungary; 14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, Sweden; International Conference on Toxicology and Risk Assessment, March 20-22, 2019 Frankfurt, Germany; Euro Summit on Toxicology and Pharmacology, November 19-21, 2018 Rome, Italy; 54th Congress of the European Societies of Toxicology 2018, September 02-05, 2018 Brussels, Belgium; Society of Environmental Toxicology and Chemistry (SETAC); IUTOX: International Union of Toxicology; EUROTOX; ABD: American Board of Toxicology
Related Societies
Europe: Society of Environmental Toxicology and Chemistry, Federation of European Toxicologists & European Societies of Toxicology (EUROTOX), British Toxicology Society (BTS), European Society of Toxicology In-Vitro.
USA : Society of Toxicology, International Association of Therapeutic Drug Monitoring and Clinical Toxicology, Fellow of the American Association for the Advancement of Science, Society of toxicologic pathology.
Asia pacific and Middle East: The Asian Society of Toxicology (ASIATOX), Asia Pacific Association of Medical Toxicology (APAMT), Chinese Society of Toxicology, Society of Toxicology India (STOX).
Track 9: Environmental Toxicology
Environmental toxicology is the study of the harmful effects of various chemical, biological and physical agents on living organisms at different levels of ecosystem. Many sources lead to environmental toxicity which includes toxicants from pollutants, insecticides, pesticides, fertilizers, industrial and metallic toxicants. All these agents have a profound influence on the living organisms. This may result in the imbalance of ecosystem.
Anthropogenic factors, manufactured chemicals etc. have a major impact on aquatic ecosystems. The first affected population due to the environmental pollutant burdens is the aquatic population. Aquatic toxicology studies the impact of these factors on the aquatic organisms at various levels of organisation and ecosystem. Aquatic toxicology is a multidisciplinary field which integrates toxicology, aquatic ecology and aquatic chemistry. The chemical substances which are used to kill, injure or incapacitate human beings with their toxic properties are called as Chemical Warfare Agents. These agents can be any state-solid, liquid, gas or volatile. These are inexpensive and relatively easy to manufacture. The chemical warfare agents may be classified into Harassing agents, Incapacitating agents, Lethal agents, Blood agents, Choking agents, Nerve agents.
Related Conferences: Toxicology Conferences | Pharmacology Conferences | Pharmaceutical Conferences
20th World Congress on Toxicology and Pharmacology, May 06-07, 2019 Tokyo, Japan; 16th Global Summit on Toxicology and Applied Pharmacology, October 15-16, 2018 Las Vegas, Nevada, USA; 15th Euro-Global Summit on Applied Pharmacology and Toxicology, July 02-04, 2018 Berlin, Germany; 17th Global Toxicology and Risk Assessment Conference, October 22-24, 2018 Budapest, Hungary; 14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, Sweden; International Conference on Toxicology and Risk Assessment, March 20-22, 2019 Frankfurt, Germany; Euro Summit on Toxicology and Pharmacology, November 19-21, 2018 Rome, Italy; 54th Congress of the European Societies of Toxicology 2018, September 02-05, 2018 Brussels, Belgium; Society of Environmental Toxicology and Chemistry (SETAC); IUTOX: International Union of Toxicology; EUROTOX; ABD: American Board of Toxicology
Related Societies
Europe: Society of Environmental Toxicology and Chemistry, Federation of European Toxicologists & European Societies of Toxicology (EUROTOX), British Toxicology Society (BTS), European Society of Toxicology In-Vitro.
USA : Society of Toxicology, International Association of Therapeutic Drug Monitoring and Clinical Toxicology, Fellow of the American Association for the Advancement of Science, Society of toxicologic pathology.
Asia pacific and Middle East: The Asian Society of Toxicology (ASIATOX), Asia Pacific Association of Medical Toxicology (APAMT), Chinese Society of Toxicology, Society of Toxicology India (STOX).
Track 10: Medical Toxicology
Medical toxicology is a subspecialty of medicine focusing on toxicology and providing the diagnosis, management, and prevention of poisoning and other adverse effects due to medications, occupational and environmental toxicants, and biological agents.
Related Conferences: Toxicology Conferences | Pharmacology Conferences | Pharmaceutical Conferences
20th World Congress on Toxicology and Pharmacology, May 06-07, 2019 Tokyo, Japan; 16th Global Summit on Toxicology and Applied Pharmacology, October 15-16, 2018 Las Vegas, Nevada, USA; 15th Euro-Global Summit on Applied Pharmacology and Toxicology, July 02-04, 2018 Berlin, Germany; 17th Global Toxicology and Risk Assessment Conference, October 22-24, 2018 Budapest, Hungary; 14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, Sweden; International Conference on Toxicology and Risk Assessment, March 20-22, 2019 Frankfurt, Germany; Euro Summit on Toxicology and Pharmacology, November 19-21, 2018 Rome, Italy; 54th Congress of the European Societies of Toxicology 2018, September 02-05, 2018 Brussels, Belgium; Society of Environmental Toxicology and Chemistry (SETAC); IUTOX: International Union of Toxicology; EUROTOX; ABD: American Board of Toxicology
Related Societies
Europe: Society of Environmental Toxicology and Chemistry, Federation of European Toxicologists & European Societies of Toxicology (EUROTOX), British Toxicology Society (BTS), European Society of Toxicology In-Vitro.
USA : Society of Toxicology, International Association of Therapeutic Drug Monitoring and Clinical Toxicology, Fellow of the American Association for the Advancement of Science, Society of toxicologic pathology.
Asia pacific and Middle East: The Asian Society of Toxicology (ASIATOX), Asia Pacific Association of Medical Toxicology (APAMT), Chinese Society of Toxicology, Society of Toxicology India (STOX).
Track 11: Medical Toxicologists work in a variety of settings including
Emergency departments and in-patient units where they directly treat acutely poisoned patients. Outpatient clinics and occupational health settings where they evaluate the health impact from exposure to toxic substances in the home or workplace. National and regional poison control centres where they provide medical direction for health professionals, personal responders and the general public
Industry and commerce where they contribute to pharmaceutical research and development, product safety, occupational health services, and regulatory compliance. Governmental agencies where they provide toxicology expertise at all levels from local health departments to federal entities. Clinical and forensic laboratories where they aid in the design, conduction and interpretation of diagnostic tests and forensic studies.
Related Conferences: Toxicology Conferences | Pharmacology Conferences | Pharmaceutical Conferences
20th World Congress on Toxicology and Pharmacology, May 06-07, 2019 Tokyo, Japan; 16th Global Summit on Toxicology and Applied Pharmacology, October 15-16, 2018 Las Vegas, Nevada, USA; 15th Euro-Global Summit on Applied Pharmacology and Toxicology, July 02-04, 2018 Berlin, Germany; 17th Global Toxicology and Risk Assessment Conference, October 22-24, 2018 Budapest, Hungary; 14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, Sweden; International Conference on Toxicology and Risk Assessment, March 20-22, 2019 Frankfurt, Germany; Euro Summit on Toxicology and Pharmacology, November 19-21, 2018 Rome, Italy; 54th Congress of the European Societies of Toxicology 2018, September 02-05, 2018 Brussels, Belgium; Society of Environmental Toxicology and Chemistry (SETAC); IUTOX: International Union of Toxicology; EUROTOX; ABD: American Board of Toxicology
Related Societies
Europe: Society of Environmental Toxicology and Chemistry, Federation of European Toxicologists & European Societies of Toxicology (EUROTOX), British Toxicology Society (BTS), European Society of Toxicology In-Vitro.
USA : Society of Toxicology, International Association of Therapeutic Drug Monitoring and Clinical Toxicology, Fellow of the American Association for the Advancement of Science, Society of toxicologic pathology.
Asia pacific and Middle East: The Asian Society of Toxicology (ASIATOX), Asia Pacific Association of Medical Toxicology (APAMT), Chinese Society of Toxicology, Society of Toxicology India (STOX).
Market Analysis
Increasing demand to curb drug toxicity at the drug development phase coupled with new drug development is expected to fuel growth of ADME toxicology testing market over the forecast period. ADME testing refers to the pharmacokinetic testing of a compound in living organism and known as ADME/Tox. Drug development is a cost and time intensive process. The traditional drug development process includes toxicity and efficacy testing in in-vivo environment which is responsible for late stage failure of drugs in human body, as the results observed in animal models and human are different. To overcome this drug failure hurdle drug manufacturers are incorporating ADME toxicity testing in early drug developmental phases. Increasing adoption of ADME toxicity testing as cost and time curbing tool is further expected to drive growth of this market. Introduction of technologically advanced computer based testing models are additionally fuelling growth of this market.
ADME toxicity testing market is studied with respect to in-vitro and in-vivo technologies. In-vitro technologies are expected to register lucrative growth owing to increasing ethical concerns over animal use in clinical trials. Insilico technologies of in-vivo testing are gaining popularity due to its capability of effective cost reduction due to early ADME toxicology prediction. Use of ADME toxicity computer modelling is expected to rise because of associated benefits related to increase throughput screening.
Based on toxicity endpoints and tests of in vitro toxicology testing, the market is sub segmented into ADME, genotoxicity, skin irritation and sensitization, cytotoxicity, ocular toxicity, organ toxicity, photo toxicity, dermal toxicity, and other toxicities like eco toxicity, endocrine disruption, and reproductive & developmental toxicity. In 2017, the ADME segment is expected to account for the largest share of the global in vitro toxicology testing market owing to its highly reproducible & accurate data and the increasing demand to curb drug toxicity at the drug development phase.
The major players in the global in vitro toxicology testing market include Cyprotex, Covance, Eurofins Scientific SE, GE Healthcare, Thermo Fisher Scientific, SGS SA, Promega Corporation, Merck KGaA, Charles River Laboratories, Inc., and Lonza Group Ltd., among others.
Major Associations around the Globe:
- Society of Toxicology, USA (SOT)
- Society of Toxicology of Canada
- Latin American Association of Toxicology (ALATOX)
- Japanese Society of Toxicology
- Italian Society of Toxicology
Target Audience:
- Professors
- PhD Scholars
- Students carrying out laboratory and field studies
- Pharmaceutical Industrial Giants
- Toxicology Societies and the people Associated
- Noble laureates in Health Care and Medicine
- Pharmacists
- Genetic Professionals
- Forensic Professionals
- Toxicology Professionals
- Pathology Professionals
- Clinical Laboratories and Technicians
- Bio instruments Professionals
- Bio-informatics Professionals
- Software development companies
- Research Institutes and members
- Supply Chain companies
- Manufacturing Companies
- Training Institutes
- German Society of Toxicology
- EUROTOX
- British Toxicology Society
- French Society of Toxicology
International Society of Regulatory Toxicology and Pharmacology
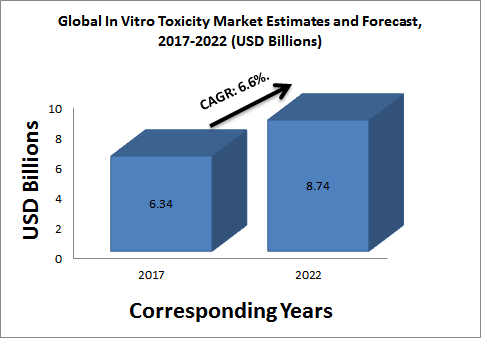
Glance at the Market:
The in vitro toxicology testing market is expected to reach USD 8.74 Billion by 2022 from an estimated USD 6.34 Billion in 2017, at a CAGR of 6.6%. The key factors driving the growth of this market include opposition to animal testing, growing demand for in vitro technology in the European market, new and promising technologies, and increasing R&D expenditure to detect toxicity at an early stage.
Figure: Global In Vitro Toxicity Market Estimates and Forecast, 2017-2022 (USD Billions)
Based on toxicity endpoints and tests of in vitro toxicology testing, the market is sub segmented into ADME, genotoxicity, skin irritation and sensitization, cytotoxicity, ocular toxicity, organ toxicity, photo toxicity, dermal toxicity, and other toxicities like eco toxicity, endocrine disruption, and reproductive & developmental toxicity. In 2017, the ADME segment is expected to account for the largest share of the global in vitro toxicology testing market owing to its highly reproducible & accurate data and the increasing demand to curb drug toxicity at the drug development phase.
The major players in the global in vitro toxicology testing market include Cyprotex, Covance, Eurofins Scientific SE, GE Healthcare, Thermo Fisher Scientific, SGS SA, Promega Corporation, Merck KGaA, Charles River Laboratories, Inc., and Lonza Group Ltd., among others.
Past Conference Report
Environment and Health Congress 2018 - Report
The World Congress on Environmental Toxicology and Health was organized during July 11-12, 2018 at the Mercure Sydney Central, Sydney, Australia. The conference was marked with the attendance of Editorial Board Members of supported Conferenceseries llc LTD Group Journals, Scientists, young and brilliant researchers, business delegates and talented student communities representing more than 20 countries, who made this conference fruitful and productive.
The conference proceeded through various Scientific Sessions and plenary lectures, of which the following topics were highlighted as Keynote presentations:
Nanomaterial carcinogenicity: Role of cancer stem cells and tumor microenvironment: Yon Rojanasakul, West Virginia University, USA
Risk assessment and bioavailability of mercury from dust in gold mining areas in Johannesburg, South Africa: Ewa Cukrowska, University of the Witwatersrand, South Africa.
The air pollution caused by wildland fires and the effects on health: Jaime Senabre, SINIF, Spain Scientific sessions were chaired by Ewa Cukrowska, University of the Witwatersrand, South Africa.
Conference Series llc LTD has taken the grand privilege of felicitating Environment and Health Congress 2018 Organizing Committee, Editorial Board Members and Keynote Speakers who supported for the success of this event.
The esteemed guests, Keynote speakers, well-known researchers, and delegates shared their innovative research and vast experience through their presentations at the podium of Environment and Health Congress 2018. We are glad to inform that all accepted abstracts for the conference have been published in the Journal of Environmental and Analytical Toxicology as a special issue.
Conference Series LLC LTD therefore is glad to announce its World Congress on Medical Toxicology, which will be scheduled during December 01-02, Kyoto, Japan. We cordially welcome all the eminent researchers, students and delegates to take part in this upcoming conference to witness eminent scientific discussions and contribute to the future innovations in the field of MEDICAL TOXICOLOGY PHARMA 2020.
To Collaborate Scientific Professionals around the World
Conference Date December 01-02, 2020
For Sponsors & Exhibitors
Speaker Opportunity
Useful Links
Supported By
All accepted abstracts will be published in respective Conference Series International Journals.
Abstracts will be provided with Digital Object Identifier by


